Összes szerző
Kania Pawel
az alábbi absztraktok szerzői között szerepel:
-
Pawel Kania (Nanotemper)
Characterize your most challenging interactions with two independent technologies. The New Monolith X. -
Aug 29 - kedd
12:30 – 12:50
Támogatói előadás
T01
Characterize your most challenging interactions with two independent technologies. The New Monolith X.
Piotr Wardega1, Pawel Kania1
1NanoTemper Technologies sp.z o.o. Kraków, Poland
pawel.kania@nanotempertech.com
Knowing the strength of the interactions between key players is crucial to get the insights you need to understand the details behind how a given biological event occurs.
The new Monolith is the latest solution we provide to our customers who wish to quantify their biomolecular interactions of any kind in any experimental conditions. Monolith utilizes two proprietary technologies- MST as well as our newest addition to the portfolio- isothermal spectral shift.
MST technology allows for quantification of molecular interactions between a target and ligand by detecting changes in fluorescence intensity while a temperature gradient is applied over time. The fluorescent signal comes from the target that is either fluorescently labeled or has intrinsic fluorescence and becomes an extremely sensitive reporter for the interaction.
Isothermal spectral shift in order to quantify a molecular interactions utilizes an experimental procedure during which a fluorescently labelled target generates a particular emission spectrum, and if a ligand binds to this target, the fluorophore’s local chemical environment is changed, causing a shift in its fluorescence spectrum. This Monolith detector exploits this phenomenon by performing ratiometric measurements at two emission wavelengths of a labelled target in the presence of various concentrations of a ligand. [1]
In both of the detectors types which can be combined in a Monolith instrument- the binding affinity is automatically determined at the end of each run without additional and lengthy data analysis. (figure 1.)
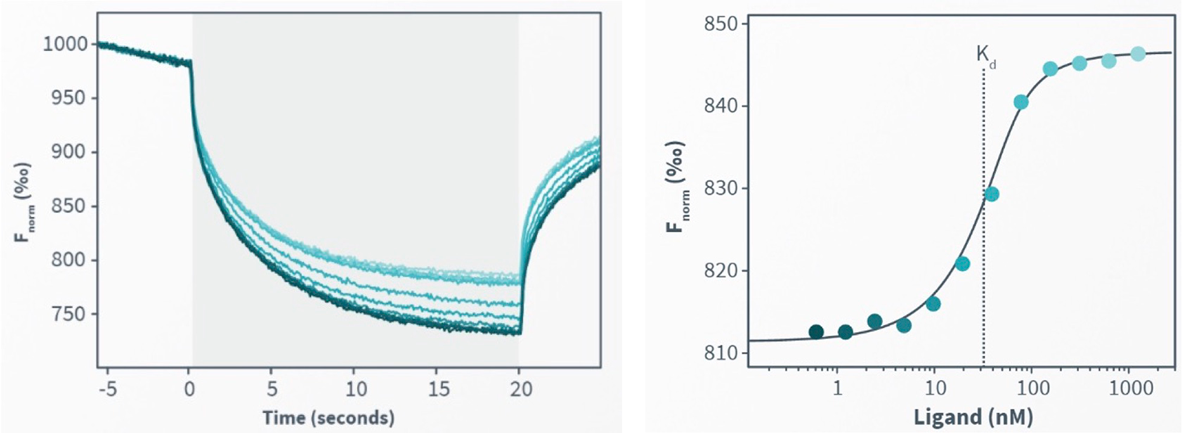
Figure 1. The affinity constant (Kd) is calculated from a fitted curve that plots normalized fluorescence against concentration of ligand.
Monolith enables characterization of in solution interactions for a wide range of biomolecules, even for challenging samples such as membrane proteins, intrinsically disordered proteins, small molecules and cell lysates. Since the binding partners are in solution, there is no lost activity due to immobilization, and evaluation is size independent. Measurements can be performed in any buffer, including detergents, using low sample volumes and concentrations. The collected data analysis also facilitates the evaluation of competition assays and ternary binding events.
Monolith provides a valuable orthogonal method to validate your results from other biophysical methods and to characterize your most challenging interactions.
References
[1] Langer A, Bartoschik T, Cehlar O, Duhr S, Baaske P, Streicher W. Assay Drug Dev Technol. 2022 Feb-Mar;20(2):83-94




